Among the more remarkable legacies of the covid-19 pandemic is how quickly federal regulators, the health care industry, and consumers moved to make at-home testing a reliable tool for managing a public health crisis.
But that fast-track focus is missing from another, less publicized epidemic: an explosion in sexually transmitted diseases that can cause chronic pain and infertility among infected adults and disable or kill infected newborns. The disparity has amplified calls from researchers, public health advocates, and health care companies urging the federal government to greenlight at-home testing kits that could vastly multiply the number of Americans testing for STDs.
Online shoppers can already choose from more than a dozen self-testing kits, typically ranging in price from $69 to $500, depending on the brand and the variety of infections they can detect.
But, except for HIV tests, the Food and Drug Administration hasn’t approved STD test kits for use outside a medical setting. That leaves consumers unsure about their reliability even as at-home use grows dramatically.
The STD epidemic is “out of control,” said Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security. “We know we are missing diagnoses. We know that contact tracing is happening late or not at all. If we’re really serious about tackling the STD crisis, we have to get more people diagnosed.”
Preliminary data for 2021 showed nearly 2.5 million reported cases of chlamydia, gonorrhea, and syphilis in the U.S., according to the Centers for Disease Control and Prevention. Reported cases of syphilis and gonorrhea have been climbing for about a decade. In its most recent prevalence estimate, the agency said that on any given day, 1 in 5 Americans are infected with any of eight common STDs.
The push to make at-home testing for STDs as easy and commonplace as at-home covid and pregnancy testing is coming from several sectors. Public health officials say their overextended staffers can’t handle the staggering need for testing and surveillance. Diagnostic and pharmaceutical companies see a business opportunity in the unmet demand.
The medical science underpinning STD testing is not particularly new or mysterious. Depending on the test, it may involve collecting a urine sample, pricking a finger for blood, or swabbing the mouth, genitals, or anus for discharge or cell samples. Medical centers and community health clinics have performed such testing for decades.
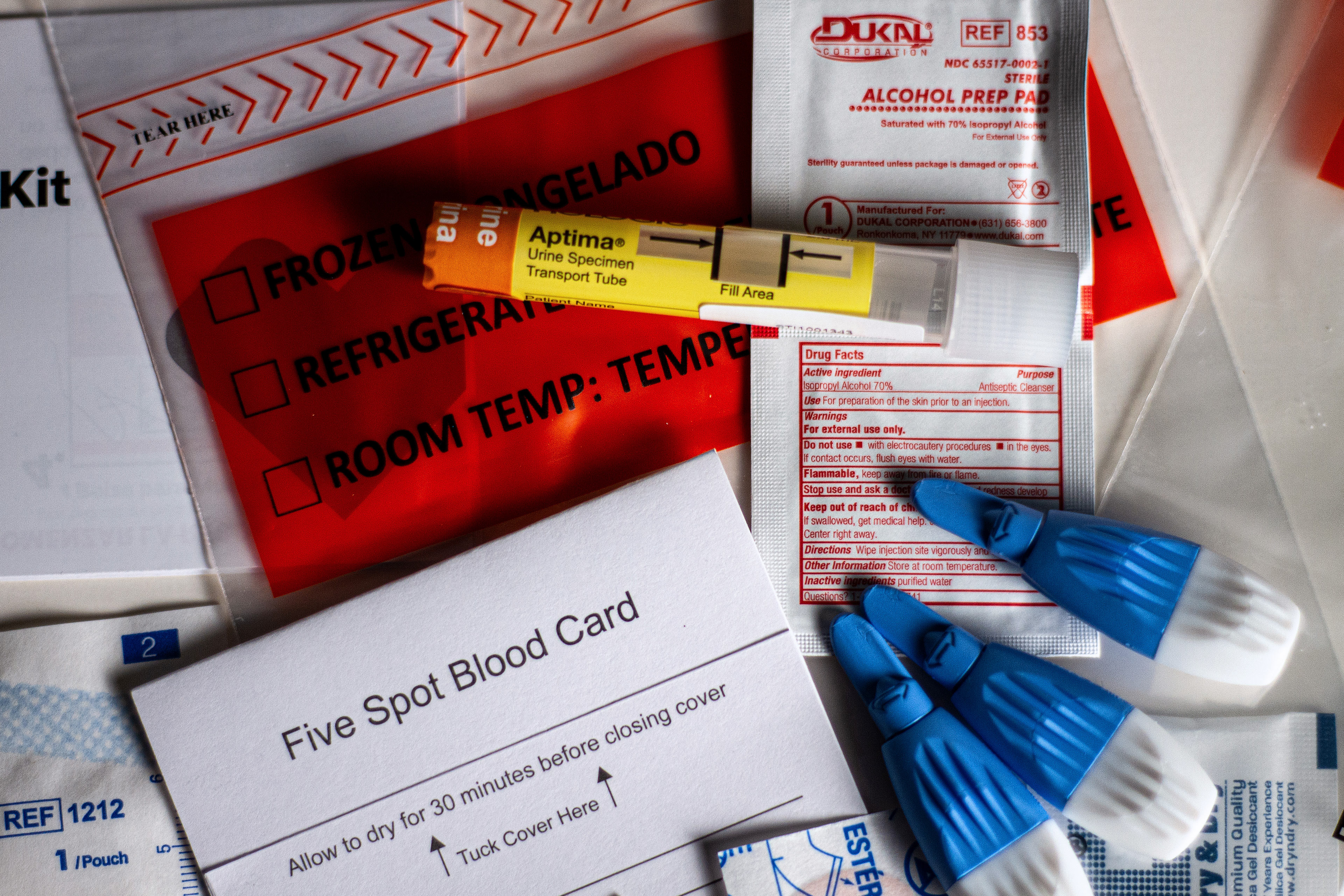
The issue for regulators is whether sampling kits can be reliably adapted for in-home use. Unlike rapid antigen tests for covid, which produce results in 15 to 20 minutes, the home STD kits on the market require patients to collect their own samples, and then package and mail them to a lab for analysis.
In the past three years, as the pandemic prompted clinics that provide low-cost care to drastically curtail in-person services, a number of public health departments — among them state agencies in Alabama, Alaska, and Maryland — have started mailing free STD test kits to residents. Universities and nonprofits are also spearheading at-home testing efforts.
And dozens of commercial enterprises are jumping into or ramping up direct-to-consumer sales. Everly Health, a digital health company that sells a variety of lab tests online, reported sales for its suite of STD kits grew 120% in the first half of this year compared with the first half of 2021.
CVS Health began selling its own bundled STD kit in October, priced at $99.99. Unlike most home kits, CVS’ version is available in stores.
Hologic, Abbott, and Molecular Testing Labs are among the companies urgently developing tests. And Cue Health, which sells covid tests, is poised to launch a clinical trial for a rapid home test for chlamydia and gonorrhea that would set a new bar, providing results in about 20 minutes.
Alberto Gutierrez, who formerly led the FDA office that oversees diagnostic tests, said agency officials have been concerned about the reliability of home tests for years. The FDA wants companies to prove that home collection kits are as accurate as those used in clinics, and that samples don’t degrade during shipping.
“The agency doesn’t believe these tests are legally marketed at this point,” said Gutierrez, a partner at NDA Partners, a consulting firm that advises companies seeking to bring health care products to market.
“CVS should not be selling that test,” he added.
In response to KHN questions, the FDA said it considers home collection kits, which can include swabs, lancets, transport tubes, and chemicals to stabilize the samples, to be devices that require agency review. The FDA “generally does not comment” on whether it plans to take action on any specific case, the statement said.
CVS spokesperson Mary Gattuso said the pharmacy chain is following the law. “We are committed to ensuring the products we offer are safe, work as intended, comply with regulations, and satisfy customers,” Gattuso said.
Everly Health and other companies described their kits as laboratory-developed tests, akin to the diagnostics some hospitals create for in-house use. And they contend their tests can be legally marketed because their labs have been certified by a different agency, the Centers for Medicare & Medicaid Services.
“The instruments and assays used by the laboratories we use are comparable to — and often the same as — those used by the labs a doctor’s office uses,” said Dr. Liz Kwo, chief medical officer at Everly Health. “Our at-home sample collection methods, like dried blood spots and saliva, have been widely used for decades.”
Home collection kits appeal to Uxmal Caldera, 27, of Miami Beach, Florida, who prefers to test in the privacy of his home. Caldera, who doesn’t have a car, said home testing saves him the time and expense of getting to a clinic.
Caldera has been testing himself for HIV and other STDs every three months for more than a year, part of routine monitoring for people taking PrEP, a regimen of daily pills to prevent HIV infection.
“Doing it by yourself is not hard at all,” said Caldera, who is uninsured but receives the tests free through a community foundation. “The instructions are really clear. I get the results in maybe four days. For sure, I would recommend it to other people.”

Dr. Leandro Mena, director of the CDC’s Division of STD Prevention, said he would like to see at-home STD testing become as routine as home pregnancy tests. An estimated 16 million to 20 million tests for gonorrhea and chlamydia are performed in the U.S. each year, Mena said. Widespread use of at-home STD testing, he said, could double or triple that number.
He noted that doctors have years of experience using home collection kits.
The Johns Hopkins Center for Point-of-Care Technologies Research for Sexually Transmitted Diseases has distributed roughly 23,000 at-home STD kits since 2004, said Charlotte Gaydos, a principal investigator with the center. The FDA generally allows such use if it’s part of research overseen by medical professionals. The center’s tests are now used by the Alaska health department, as well as Native American tribes in Arizona and Oklahoma.
Gaydos has published dozens of studies establishing that home collection kits for diseases such as chlamydia and gonorrhea are accurate and easy to use.
“There’s a huge amount of data showing that home testing works,” said Gaydos.
But Gaydos noted that her studies have been limited to small sample sizes. She said she doesn’t have the millions of dollars in funding it would take to run the sort of comprehensive trial the FDA typically requires for approval.
Jenny Mahn, director of clinical and sexual health at the National Coalition of STD Directors, said many public health labs are reluctant to handle home kits. “The public health labs won’t touch it without FDA’s blessing,” Mahn said.
Public health clinics often provide STD testing at little to no cost, while health insurance typically covers in-person testing at a private practice. But most consumers pay out-of-pocket for direct-to-consumer kits. Commercial pricing puts them out of reach for many people, particularly teens and young adults, who account for nearly half of STDs.
Adalja, at Johns Hopkins, said the FDA has a history of moving slowly on home testing. The agency spent seven years evaluating the first home HIV test it approved, which hit the market in 2012.
“Home testing is the way of the future,” said Laura Lindberg, a professor of public health at Rutgers University. “The pandemic opened the door to testing and treatment at home without traveling to a health care provider, and we aren’t going to be able to put the genie back in the bottle.”


0 Commentaires